

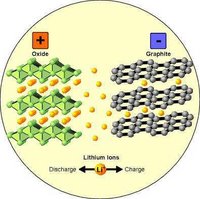
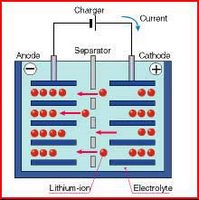
 The lithium ion (Li ion) battery, left graphics, is a rechargeable battery technology introduced in 1991 that provides greater charge per pound than nickel metal hydride. In 1993, Toshiba introduced the first notebook in the U.S. with a Li-ion battery. Since then, it has become the most popular battery technology for notebooks, cellphones and other handheld devices. In contrast to nickel-based batteries that require full discharges to keep the battery healthy, lithium ion batteries are better with frequent, shallow discharges before charging again.
The lithium ion (Li ion) battery, left graphics, is a rechargeable battery technology introduced in 1991 that provides greater charge per pound than nickel metal hydride. In 1993, Toshiba introduced the first notebook in the U.S. with a Li-ion battery. Since then, it has become the most popular battery technology for notebooks, cellphones and other handheld devices. In contrast to nickel-based batteries that require full discharges to keep the battery healthy, lithium ion batteries are better with frequent, shallow discharges before charging again.Lithium is the third lightest element, giving a substantial saving in weight compared to batteries using much heavier metals. lithium-ion batteries should be charged early and often. Li-ion batteries should be kept cool. Ideally they are stored in a refrigerator. Aging will take its toll much faster at high temperatures. The high temperatures found in cars cause lithium-ion batteries to degrade rapidly. Lithium-ion batteries should never be depleted to empty (0%). Lithium-ion batteries can easily rupture, ignite, or explode when exposed to high temperatures, or direct sunlight. They should not be stored in a car during hot weather. Short-circuiting a Li-ion battery can cause it to ignite or explode. Never open a Li-ion battery's casing. Li-ion batteries contain safety devices that protect the cells inside from abuse. If damaged, these can also cause the battery to ignite or explode.
Applications of the Nickel Metal (Cadmium) Hidride (NiMH) type battery, top pictures, includes hybrid vehicles such as the Toyota Prius and consumer electronics. NiMH battery technology was invented developed at the end of the 1980's and commercialised first by Matsushita Company. (Wikipedia, Answers)
No comments:
Post a Comment