The first four alkanes are methane, ethane, propane, and butane with the Lewis symbols shown below.
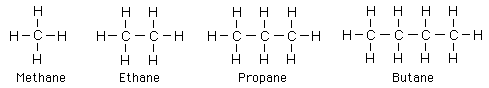
Past this number of carbons, the -ane suffix is retained and the number prefixes penta-, hexa-, hept-, oct-, non-, dec-, etc are used. Alkyl groups are used as substituents, and alkane derivatives have many applications.
The alkanes are highly combustible and are valuable as clean fuels, burning to form water and carbon dioxide. Methane, ethane, propane and butane are gases and used directly as fuels. Alkanes from pentane up to around C17H36 are liquids. Gasoline is a mixture of alkanes from pentane up to about decane. Kerosene contains alkanes from about n=10 to n=16. Above n=17 they are solids at room temperature. Alkanes with higher values of n are found in diesel fuel, fuel oil, petroleum jelly, paraffin wax, motor oils, and for the highest values of n, asphalt.
Alkane derivatives are used in hundreds of products such as plastics, paints, drugs, cosmetics, detergents, insedticides, etc., so the fossil fuel resource from which we obtain the alkanes is much too valuable to burn it all as a motor fuel. (Hyperphysics)

No comments:
Post a Comment